By Deon Canyon, Sebastian Kevany and Michael S. Baker *
Mutations are caused by random, spontaneous errors in the RNA-based genetic code of viruses that occur as the virus replicates within a host. The process of continually emerging, small mutations is called antigenic drift. In influenza and Covid, these mutations are often noticeable by observing changes in viral surface proteins, otherwise known as antigens. The human immune system identifies and reacts to foreign antigens by producing antibodies that target the infection. Most vaccines work by presenting a harmless version of the foreign antigen to train the immune system to be able to recognize and respond to the real virus. Table 1 shows the current Covid variants and mutations of concern and interest.
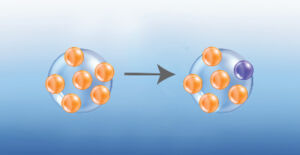 The small antigenic drift changes result in the gradual evolution of viruses into phylogenetic trees, and it is the reason why a new flu vaccine is required each year. The immune system provides cross-protection when it recognizes a similar Covid virus that contains mutations. The accumulation of mutations may differentiate a virus to the extent that it creates a different branch on the phylogenetic tree and is called a variant (image right). When a variant moves into a population that has not yet experienced it, cross-protection may not be as effective and illness may result.
The small antigenic drift changes result in the gradual evolution of viruses into phylogenetic trees, and it is the reason why a new flu vaccine is required each year. The immune system provides cross-protection when it recognizes a similar Covid virus that contains mutations. The accumulation of mutations may differentiate a virus to the extent that it creates a different branch on the phylogenetic tree and is called a variant (image right). When a variant moves into a population that has not yet experienced it, cross-protection may not be as effective and illness may result.
Variants may behave in different ways depending on the types of mutations that have occurred. Variants may have different transmissibility, morbidity, mortality, ability to detect with tests, susceptibility to antiviral drugs, susceptibility to neutralizing antibodies, and ability to evade natural immunity. This might allow them to cause recurrent infections, allow them to infect vaccinated individuals, result in long-term morbidity, or show an affinity for particular demographic groups, such as children or immunocompromised individuals. The implication for influenza is that global surveillance is necessary to identify new variants for designing new annual vaccines. While Covid does not mutate as fast as influenza, its significant mutations require global surveillance and perhaps even ongoing vaccine revisions.
Viruses typically show little variation when it comes to factors that impact the severity of human infection and transmissibility, however, they may decrease or increase in certain factors that are relevant to health security. For instance, the B.1.1.7 variant has 22 coding changes in its genome and is up to 70% more transmissible.
Most mutations in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are inconsequential, but one emerged in the United Kingdom that is more transmissible and two emerged in Brazil and South Africa that are able to escape the human immune response, even though vaccines are still effective against them. These variants are responsible for infection surges in many countries and already exist in the United States. If they build to critical mass before enough vaccinations can stop the spread, similar case surges will be seen in the U.S.
Table 1: Covid variants and mutations of concern and interest in the US.*
| Variants of Concern/ Interest | Mutations of Concern/Interest | Average Daily Prevalence US/world (nations with cases) |
|---|---|---|
| B.1.1.7 Emerged 09/20 U.K. |
|
US 54% stable World 78% growth 119 nations |
| B.1.351 Emerged 07/20 South Africa |
|
US 0% decline World 2% growth 81 nations |
| P.1 Emerged 12/20 Brazil |
|
US 6% stable World 3% growth 40 nations |
| B.1.427 Emerged 09/20 California | US 1% decline World 0% decline 29 nations |
|
| B.1.429 Emerged 07/20 California |
|
US 1% decline World 1% decline 30 nations |
| B.1.526.1 Emerged 10/20 New York |
|
US 2% decline World 1% decline 15 nations |
| B.1.526.2 Emerged 12/20 New York |
|
US 6% growth World 2% stable 6 nations |
| B.1.526 Emerged 09/20 New York |
|
US 5% decline World 1% decline 19 nations |
| B.1.617 Emerged 09/20 India |
|
US 0% decline World 1% growth 6 nations |
| P.2 Emerged 04/20 Brazil |
|
US/World 0% Brazil 3% decline 32 nations |
*Outbreak.info (data from April 26, 2021)
Viruses also experience abrupt and large mutations, where many nucleotides and genes are changed in a process called antigenic shift (image right). Typically, antigenic shift occurs when two different types of virus are found infecting a single host cell. In the process of RNA replication, genetic cross-over occurs resulting in new viruses with mixed genomes. Two examples are the 1957 Asian Influenza outbreak resulting from a cross between the 1918 Spanish Flu virus and the H2N2 Avian virus; and the 1968 Hong Kong Influenza outbreak resulting from a cross between the 1957 virus and the H3 Avian virus.*Outbreak.info (data from April 26, 2021)
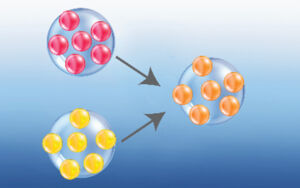 Thus, antigenic shift is one explanation for how a virus jumps from thriving in a particular animal to thriving in humans. The new mixed virus is so different from what people have experienced in the past that their immune systems fail to recognize the pathogen and serious disease may occur. Antigenic shift is rare, for there have only been four influenza pandemics in the last century.
Thus, antigenic shift is one explanation for how a virus jumps from thriving in a particular animal to thriving in humans. The new mixed virus is so different from what people have experienced in the past that their immune systems fail to recognize the pathogen and serious disease may occur. Antigenic shift is rare, for there have only been four influenza pandemics in the last century.
Covid Mutations
While Covid was still in China, the D614G mutation appeared and quickly became dominant. In this environment, a “burst in changes in the virus you wouldn’t otherwise see” may have happened in areas where cases were out of control and there were higher levels of community transmission. Wuhan city has an incredibly dense population of 10,000-90,000 people/km2 that would have facilitated this process. Within 5 to 10 months, several significant Covid mutations evolved and spread (Table 1).
The first international variant of concern (B.1.351), emerged in Nelson Mandela Bay, South Africa in July 2020. It spread faster, was detected more often in young people, and more frequently caused serious disease. The population is relatively young with only 6% aged over 64 years old. However, the highest population density was 500-3000/km2. Likewise, when a variant of concern (P.1) emerged in Manaus in the Brazilian Amazonas region in December 2020, it had a similar demographic with only 6% of people over 60 years of age and a low population density of 191/km2. The outbreak in Manaus was suspected to have been accelerated by dense housing, poor water supplies, and crowding on boats that serve as local transportation. It is thought that in these conditions, a very high infection prevalence of 76% resulted in a high level of natural immunity which selected virus mutations that could neutralize antibodies.
The second international variant of concern (B.1.1.7) emerged in September 2020 and was detected in December when restrictions did not reduce cases as expected in County Kent, United Kingdom. This variant is well on its way to becoming globally dominant. Urban areas in Kent County range in population density from 5000 to 10000/km2, but the living conditions are far different from those in Nelson Mandella Bay and Manaus. In this environment, the variant is thought to have mutated over a long time (2-4+ months) in chronically infected immunocompromised patients. Multiple mutations are more likely to develop in response to antibody or convalescent plasma treatment in immunocompromised patients with persistent infection.
More frequent antigenic drift mutations are expected when a virus is under selection pressure from natural and vaccine conferred immunity. As we exert more pressure on Covid, more virulent mutations will evolve that could directly impact our ability to manage the pandemic. The presence of large numbers of unvaccinated people only benefits this process of mutation, since it provides a fresh uninfected group of people in which the virus can spread and mutate further. On a positive note, the current U.S. administration has just funded a major expansion in virus tracking and gene sequencing.
Future Covid Threats
The emergence of Covid variants of concern and unvaccinated segments of the population have the following ongoing implications for national security and security sectors in general.
- If large segments of the global population remain without immunity, they provide a petri dish for mutation
- Governments need to accelerate and enforce vaccination policies at every opportunity
- Campaigns against misinformation must be led by a government partnership with tech companies
- Better funding and more coordination between U.S. CDC and National Center for Medical Intelligence (NCMI) along with military forward deployed labs such as Navy Medical Research Units (NAMRU) will help to provide an early warning biodefense shield
- Timely warning and projection of significant infectious disease and environmental health threats will be enhanced by strengthening ties with U.S. allies and partner nations to form a virus surveillance network
- S. intelligence agencies need to ramp up medical threat surveillance capabilities
Perhaps the most important consequence of mutations for international security dynamics is that governments would be potentially remiss in turning away from health security protocols and issues, including funding for variant testing, even after the acute phase of the global pandemic is over. The temptation to reallocate scare resources away from public health efforts in the post-pandemic period is, though, inevitably going to be a strong and equally compelling one.
A further argument in favor of increased testing for variants relates to the news media and the high level of paranoia that can result from news stories on new strains. With greater funding for variant monitoring and testing, fact checks on such stories can be easily and quickly verified from independent and objective sources, thereby bringing peace of mind to society.
None of the above suggests reduced funding and support of vaccine roll-outs, which are and should rightly be, the top public health priority worldwide. Only with the US and other developed countries assisting the developing world, in fact, can the threat of new mutations and variants be restricted. The longer that the developed world goes without comprehensive vaccination programs, the greater the risk of rampant infections and further mutation.
*Drs. Canyon and Kevany are professors at the Daniel K. Inouye Asia-Pacific Center for Security Studies (DKI APCSS) in Honolulu, USA. Dr. Baker is a retired U. S. Navy rear admiral. The views expressed in this article are the author’s alone, and do not necessarily reflect the official position of the DKI APCSS or the United States Government.
The views expressed in this article are the author’s alone, and do not necessarily reflect the official position of the DKI APCSS or the United States Government. May 2021
Published: May 8, 2021
Category: Perspectives
Volume: 22 - 2021





